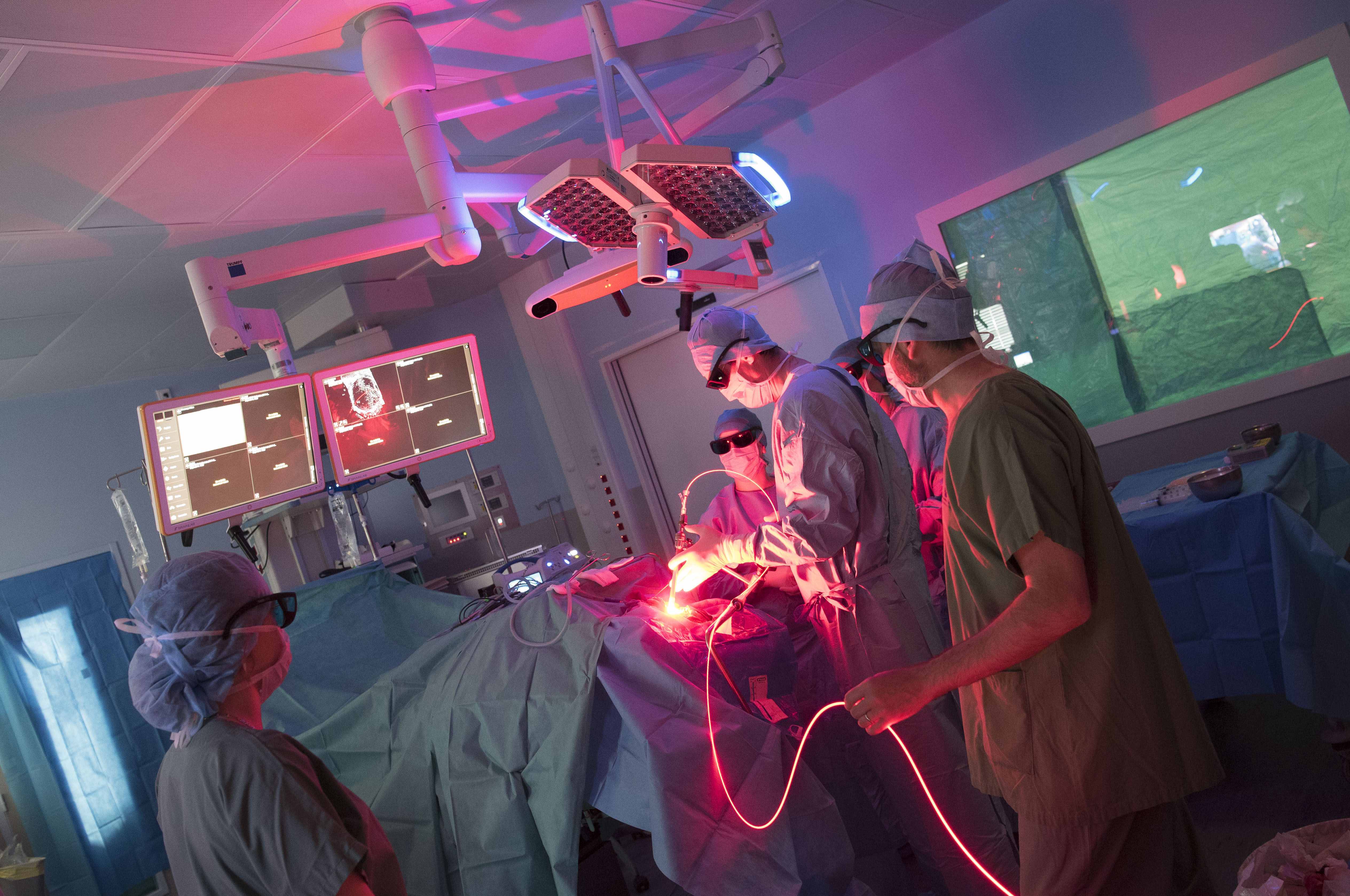
A pilot study to assess feasibility and safety of intraoperative 5-aminolevulinic acid mediated photodynamic (5-ALA PDT) therapy for the treatment of newly diagnosed glioblastoma (GBM) |
Ongoing / closed: Active (enrollment completed, patients follow-up) Research type (rétro/prospective): Prospective |
|
Synopsis: Glioblastoma is the most infiltrative and aggressive primitive cerebral tumour (grade IV, WHO). Despite the armamentarium, including procedures such as maximal microsurgical resection followed by concomitant radio-chemotherapy following maintenance chemotherapy, patients with glioblastoma still experience a dismal prognosis, with a median survival of 15 months. Due to the invasive growth characteristics, 85% of GBM recurrence occurs within 2.5 cm of the postsurgical cavity. Thus, it is critical to improve local control of the disease. In this context, photodynamic therapy (PDT), using 5-aminolevulinic acid (5-ALA) - or talaporfin sodium-induced fluorescence, may be effective.
|
|
Principal Investigator: Pr. Nicolas Reyns (Neurosurgery Dpt - University Hospital of Lille - France) |
|
Scientific coordinators: Dr. Maximilien Vermandel (University of Lille - France) - Pr. Serge Mordon (INSERM - France) |
|
Study Sponsor: University Hospital of Lille - France |
|
Primary endpoint: feasibility study of intraoperative photodynamic therapy early after surgical resection of glioblastoma without unacceptable and unexpected toxicities |
|
Secondary endpoints:
|
Evaluation criteria of the primary endpoint: Assessment of the feasibility of intraoperative PDT (after 4 hours preoperative administration of 5-ALA and maximum surgical resection of the tumor bed), with a collection of unacceptable and unexpected toxicities ≥ Grade 3 (according to NCI-CTC V4.0) reviewed by a Independent Safety Committee up to 1 month after PDT. |
|
Evaluation criteria of the secondary endpoints:
|
Estimated enrollment: 10 patients |
|
Clinicaltrials.gov identifier: NCT03048240 |
|
Contact and location: University Hospital of Lille, France
|